Environment & Energy
Related: About this forumSeparations of Actinides and Lanthanides From Used Nuclear Fuel with Destructible Ligands.
As the United States commits scientific suicide in the reign of its idiot king installed by the weakest minds with the lowest moral sense, work on saving the world goes on in the rest of the world. The paper I'll discuss in this post is from the nation that now leads in nuclear innovation, China, given the deliberate destruction of American scientific strength by uneducated incompetent fascist fools running our government for the purpose of destroying it.
A key resource for saving the world is used nuclear fuel, which contains many valuable materials, among them the transuranium actinides neptunium, plutonium, americium, and curium, along with some higher actinides. The transuranium actinides are always formed in the presence of their formal congeners the lanthanides (aka "rare earth" elements), particularly the sub-gadolinium lanthanides from lanthanum up to an including gadolinium. The separation of plutonium, neptunium and uranium from the lanthanides has been industrially practiced for decades, but (as I'm working to inform my son) increasingly available transplutonium actinides, americium and curium. I believe these two elements offer some properties that make them important resources for a sustainable world, particularly given their exceptionally valuable neutronics.
The paper to which I'll refer in this post is this one:
Efficient Trivalent Actinide-Lanthanide Separations Using Hydrophilic CHON-Compliant Phenanthroline-Dicarboxamide Ligands Yong Qiang Wan, Huai Xin Hao, Yang Yang Zhang, Yu Xiao Guo, Zhi Jun Ma, Zhi Peng Wang, Jun Li, and Pavle Mocilac Industrial & Engineering Chemistry Research 2025 64 (24), 12185-12199.
From the introduction:
There are several classes of hydrophilic ligands developed for the i-SANEX process, which include sulfonated bistriazinyl-pyridines (SO3–BTP), (7) sulfonated bistriazinyl-bipyridines (TS-BTBP), (8) bistriazinyl-phenanthrolines (TS-BTPhen), (8,9) and bistriazolyl-phenanthrolines (10) (DS-BTrzPhen). Their ability to strip minor actinides from DIAMEX raffinate is satisfactory for most of them, while the disadvantage of sulfonated ligands is not being CHON-compliant. On the other hand, the number of promising CHON-compliant ligands is limited and includes primarily bistriazolyl-pyridines such as PTD. (11,3) In this context, “CHON” refers to ligands composed solely of carbon, hydrogen, oxygen, and nitrogen. This criterion is significant because the subsequent processing of extracts containing americium (Am) and curium (Cm) complexed with these ligands will involve solvent evaporation and thermal oxidation. During this process, C, H, O, and N will combust into gaseous oxides, leaving behind only metal oxides. The presence of other elements would complicate the final stages of reprocessing and result in unnecessary secondary waste. (12)
A crucial category of CHON-compliant ligands is the 1,10-phenanthroline-dicarboxamides, commonly referred to as diamide-phenanthrolines (Figure 1a) or “DAPhens”. (13) These ligands operate on the hard–soft (O/N) donor concept. Initially developed for the SANEX process, DAPhens were designed to be lipophilic, while their development was inspired by dipicolinic acid diamides by adapting the “hard–soft” donor concept from pyridine to phenanthroline. (14) A landmark study published in 2014 by Xiao et al. introduced Et-Tol-DAPhen (Figure 1b), which demonstrated favorable separation factors for actinides compared to europium (Eu) (SFAn/Eu) in acidic media. (15) While DAPhens exhibited moderate SFAn/Ln values in conventional solvents, (16) they demonstrated improved efficiency in 1,2-dichloroethane (17) or nitrobenzene, (18,19) while in 1-(trifluoromethyl)-3-nitrobenzene (F-3) the SFAn/Ln values reached excellent levels, ranging from 600 to 700. (20−22) DAPhens were also tested in ionic liquids, however without significant improvement in selectivity. (23,24) Derivatives of DAPhen with 4,7-dichloro (25,26) and fluoro (27) functional groups on the phenanthroline core moiety were also developed (Figure 1c)...(28)
Structures of ligands:
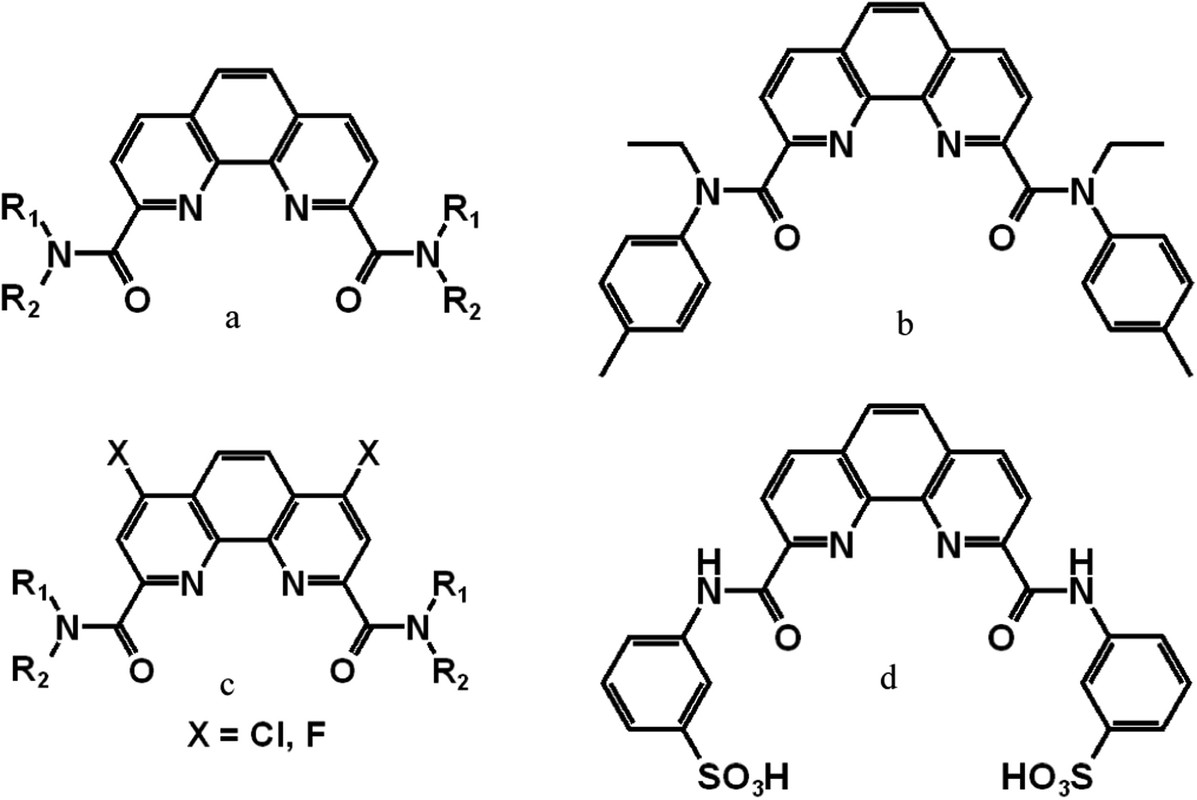
The caption:
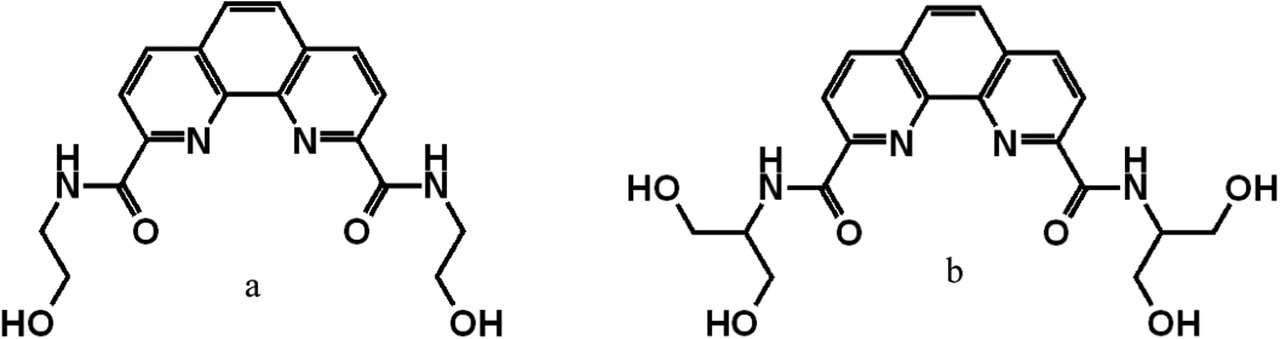
The caption:
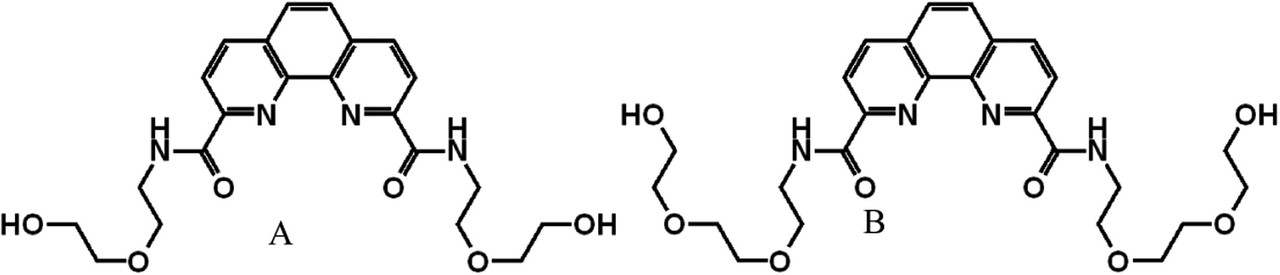
The caption:
The paper goes into the synthetic details of these ligands, and then describes a number of experiments to determine the separation factors (SF) for lanthanide species (focusing on europium in many cases) and the actinides curium and americium. I note that these two actinides, unlike the other actinides in used nuclear fuel, uranium (which dominates the mass), neptunium and plutonium, do not form volatile fluorides, and thus will remain with those fission products which cannot be volatilized, consisting largely of the lanthanides. Thus I see this process as synergetic with the important fluoride volatility residues.
There are a lot of graphics showing separation factors under various conditions, and I will not produce most of them, but offer this sample:
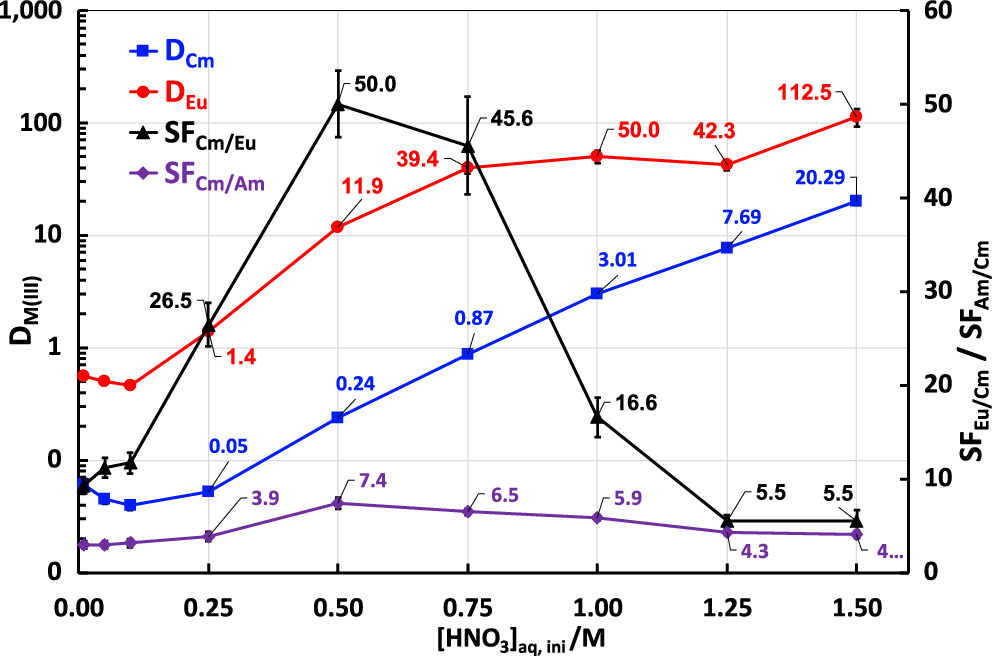
The caption:
The separation of curium from americium is relatively modest, but one could argue that it would be OK - even in some cases desirable - to carry some curium - as a source of spontaneous fission neutrons - in an americium based nuclear fuel.
The paper contains some wonderful scientific details, including molecular orbital discussions related to the binding, but they are superfluous to discuss here.
Some additional commentary from the authors:
We thoroughly investigated the ability of the two ligands, 1 and 2, to separate Am(III) ions from Eu(III) ions using two different methods: masking and stripping. Both methods yielded similar excellent results for SFEu/Am of 201–210 at 0.5 M acidity. However, increasing the ligand concentration to 30 mM brings the SFEu/Am values to 290–325 even at a 0.75 M acidity. The lower separation factors for ligand 2 (AEE-DAPhen) can be considered as evidence that the presence of hydrophilizing ethylene-glycol chains may interfere with the selectivity and extraction characteristics of these ligands. In fact, poly(ethylene-glycol) chains were so far avoided as hydrophilizing groups due to their ability to interfere with the coordination process. Our theoretical study herein suggests that ethylene-glycol chains do engage in coordination with lanthanides, whereas it could be assumed that the interaction in the case of ligand 2 could be more pronounced due to the flexibility of the chains. Yet, there is no crucial evidence that the presence of these chains is detrimental to selectivity toward Am(III) ions since ligand 2 can still be regarded as a selective ligand.
From the paper's conclusion:
It's a nice paper to see. The key to saving the world is mass efficient use of actinide resources, especially 238U, so called "depleted uranium" by converting it to plutonium and higher actinides.
Even though the United States is a free fall stemming from its long history of and enthusiasm for racism, the rest of the world can go on to do the necessary tasks we have abrogated.
Have a nice weekend.